Multiple Choice
What is the percent ionization of nitrous acid in a solution that is 0.222 M in nitrous acid (HNO2) and 0.278 M in potassium nitrite (KNO2) ? The acid dissociation constant of nitrous acid is 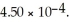
A) 55.6
B) 15.5
C) 2.78 × 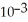
D) 3.448
E) 0.162
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q18: The Henderson-Hasselbalch equation is _.<br>A)[H<sup>+</sup>] = K<sub>a</sub>
Q19: Consider a solution containing 0.100 M fluoride
Q20: A 25.0 mL sample of a solution
Q21: A 25.0-mL sample of 0.150 M hydrocyanic
Q22: For which salt should the aqueous solubility
Q24: Which one of the following is not
Q25: Which one of the following pairs cannot
Q26: In a solution,when the concentrations of a
Q27: Decreasing the pH of blood will cause
Q28: The K<sub>sp</sub> for Cu(OH)<sub>2</sub> is 4.8 ×