Multiple Choice
What is the pH of a buffer solution that is 0.266 M in lactic acid and 0.111 M in sodium lactate? The 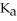 of lactic acid is 1.4 ×
of lactic acid is 1.4 × 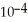 .
.
A) 14.38
B) 10.53
C) 5.38
D) 3.47
E) 4.23
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q101: Why does fluoride treatment render teeth more
Q102: What is the pH of a solution
Q103: A solution of NaF is added dropwise
Q104: What is the solubility (in M)of PbCl<sub>2</sub>
Q105: Calculate the percent ionization of formic acid
Q107: Which of the following could be added
Q108: In general,the solubility of a slightly soluble
Q109: The pH of a solution prepared by
Q110: What is the pH of a buffer
Q111: What is the pH of a solution