Multiple Choice
What is the pH of a solution that contains 0.800 M weak acid ( 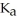 = 1.76 ×
= 1.76 × 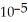 ) and 0.172 M of its conjugate base?
) and 0.172 M of its conjugate base?
A) 8.578
B) 5.422
C) 8.370
D) 4.087
E) 9.913
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q71: Metal oxides and hydroxides that are relatively
Q72: The <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="The of
Q73: What is the primary buffer system that
Q74: A complex ion is when a metal
Q75: A 25.0 mL sample of an acetic
Q77: Which one of the following pairs cannot
Q78: Suppose you have just added 100.0 ml
Q79: Suppose you have just added 50.0 ml
Q80: In which of the following aqueous solutions
Q81: The solubility of manganese (II)hydroxide (Mn(OH)<sub>2</sub>)is <img