Multiple Choice
Cl atoms formed via photolysis of C-Cl bonds of chlorofluorocarbons in the stratosphere are particularly effective in destroying ozone at these altitudes because ________.
A) Cl atoms absorb UV, which generate O atoms to react with 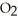 to produce ozone
to produce ozone
B) Cl atoms catalytically convert O3 to O2
C) Cl atoms stoichiometrically convert O3 to O2
D) Cl atoms react with H atoms, which catalyze conversion of O2 to O3
E) Cl atoms react with N atoms, which catalyze conversion of O2 to O3
Correct Answer:

Verified
Correct Answer:
Verified
Q69: The salinity,density,and temperature of seawater varies with
Q70: Sulfur released into the troposphere arises from
Q71: The C <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="The C
Q72: THMs are _.<br>A)non-toxic<br>B)natural<br>C)used in green chemistry<br>D)suspected carcinogens<br>E)atmospheric
Q73: The world's largest desalinization plant is in
Q75: Why does the upper atmosphere contain only
Q76: Which one of the following substances found
Q77: CO<sub>2</sub> from hydrocarbon combustion creates a major
Q78: Photoionization processes (e.g.,N<sub>2</sub> + hν → N<sub>2</sub><sup>+</sup>
Q79: The contribution of _ to acid rain