Multiple Choice
How does lime reduce sulfur dioxide emissions from the burning of coal?
A) It reacts with the sulfur dioxide to form calcium sulfite solid that can be precipitated.
B) It reduces the sulfur dioxide to elemental sulfur that is harmless to the environment.
C) It oxidizes the sulfur dioxide to tetrathionate that is highly water soluble so it can be scrubbed from the emission gases.
D) It catalyzes the conversion of sulfur dioxide to sulfur trioxide which is much less volatile and can be removed by condensation.
E) It converts S 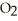 to solid, elemental sulfur.
to solid, elemental sulfur.
Correct Answer:

Verified
Correct Answer:
Verified
Q4: The average pH of the oceans is
Q5: What is the percentage of freshwater on
Q6: The reaction that forms most of the
Q7: Which of the following is classified as
Q8: The concentration of CO in a room
Q10: Radiation in the infrared portion of the
Q11: Chemical treatment of municipal water supplies commonly
Q12: The majority of SO<sub>2</sub> released annually in
Q13: In the world's oceans,the average salinity is
Q14: Natural,unpolluted rainwater is typically acidic.What is the