Multiple Choice
What is the total pressure (torr) inside a container that contains a gas at 3.18 × 103 ppm where its partial pressure is 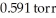 ?
?
A) 5.38 × 10-3
B) 1.86 × 10-4
C) 1.88 × 103
D) 1.86 × 10-10
E) 1.88 × 109
Correct Answer:

Verified
Correct Answer:
Verified
Q51: In the troposphere,temperature _ with increasing altitude,while
Q52: What is/are the product(s)of photodissociation of molecular
Q53: In the equation below,M is most likely
Q54: Ozone depletion from chlorofluorocarbons is chiefly due
Q55: What are the primary chemical pollutants that
Q57: The mole fraction of nitrous oxide in
Q58: Acid rain typically has a pH of
Q59: Show how a molecule of C<sub>2</sub>F<sub>2</sub>Cl<sub>2</sub> can
Q60: The cumulative result of carbon dioxide,methane,and ozone
Q61: As one gains altitude in the atmosphere,based