Multiple Choice
What is the concentration (ppm) of water vapor in a sample of air that has a partial pressure of water of 1.20 torr and a total pressure of air of 695 torr?
A) 1.7
B) 0.17
C) 5.8 × 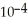
D) 1.7 × 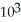
E) 0.58
Correct Answer:

Verified
Correct Answer:
Verified
Q39: Compounds found in fossil fuels that contain
Q40: CFC stands for _.<br>A)chlorinated freon compound<br>B)chlorofluorocarbon<br>C)carbonated fluorine
Q41: Municipal water treatment consists of five steps
Q42: Nitric oxide arises from auto emissions and
Q43: Which of the following is (are)not a
Q45: The total pressure of a component in
Q46: Of the following,only _ does not result
Q47: Components of Air Mole Fraction Nitrogen 0.781<br>Oxygen
Q48: The mole fraction of nitrogen in dry
Q49: The concentration of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="The concentration