Multiple Choice
The concentration of carbon monoxide in a sample of air is 6.0 ppm.There are ________ molecules of CO in 1.00 L of this air at 755 torr and 23 °C.
A) 2.5 × 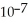
B) 1.4 × 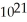
C) 1.9 × 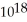
D) 1.1 × 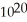
E) 1.5 × 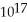
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q11: Chemical treatment of municipal water supplies commonly
Q12: The majority of SO<sub>2</sub> released annually in
Q13: In the world's oceans,the average salinity is
Q14: Natural,unpolluted rainwater is typically acidic.What is the
Q15: Components of Air Mole Fraction Nitrogen 0.781<br>Oxygen
Q17: The absorption of a photon which results
Q18: The layer of the atmosphere that contains
Q19: Of the reactions involved in the photodecomposition
Q20: How can the presence of biodegradable waste
Q21: The most abundant component of air is