Multiple Choice
Given the following table of thermodynamic data,  complete the following sentence.The vaporization of Ti
complete the following sentence.The vaporization of Ti 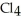 is ________.
is ________.
A) spontaneous at all temperatures
B) spontaneous at low temperature and nonspontaneous at high temperature
C) nonspontaneous at low temperature and spontaneous at high temperature
D) nonspontaneous at all temperatures
E) not enough information given to draw a conclusion
Correct Answer:

Verified
Correct Answer:
Verified
Q31: The value of ΔS° for the decomposition
Q32: The value of ΔS° for the oxidation
Q33: The vaporization of a substance at its
Q34: The thermodynamic quantity that expresses the extent
Q35: Which reaction produces a decrease in the
Q37: Which one of the following is always
Q38: Which of the following has the largest
Q39: Which one of the following processes produces
Q40: The value of ΔH° for the decomposition
Q41: ΔS is positive for the reaction _.<br>A)2H<sub>2</sub>