Multiple Choice
Consider the reaction between ammonia and hydrochloric acid to produce ammonium chloride. Given the following table of thermodynamic data at 298 K: 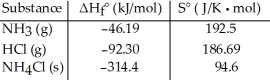 The value of K for the reaction at 25 °C is ________.
The value of K for the reaction at 25 °C is ________.
A) 8.4 × 104
B) 150
C) 1.1 × 10-16
D) 9.3 × 1015
E) 1.4 × 108
Correct Answer:

Verified
Correct Answer:
Verified
Q20: Calculate ΔG°<sup> </sup>(in kJ/mol)for the following reaction
Q21: The value of ΔS° for the oxidation
Q22: Consider the formation of solid silver chloride
Q23: The entropy of a pure crystalline substance
Q24: Which of the following statements is false?<br>A)The
Q26: For the reaction C<sub>2</sub>H<sub>6</sub> (g)→ C<sub>2</sub>H<sub>4</sub> (g)+
Q27: The value of ΔS° for the oxidation
Q28: The value of ΔG° at 25 °C
Q29: The value of ΔS° for the formation
Q30: ΔS is positive for the reaction _.<br>A)2NO