Multiple Choice
The normal boiling point of methanol is 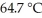 and the molar enthalpy of vaporization if 71.8 kJ/mol.The value of ΔS when 1.75 mol of
and the molar enthalpy of vaporization if 71.8 kJ/mol.The value of ΔS when 1.75 mol of 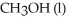 vaporizes at 64.7 °C is
vaporizes at 64.7 °C is 
A) 0.372
B) 372
C) 1.94 × 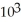
D) 4.24 × 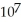
E) 1.94
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q6: ΔS is positive for the reaction _.<br>A)CaO
Q7: For an isothermal process,the entropy change of
Q8: The value of ΔG° at 25 °C
Q9: Calculate ΔG<sup>∘ </sup>(in kJ/mol)for the following reaction
Q10: The value of ΔH° for the formation
Q12: The value of ΔG° at 181.0 °C
Q13: The value of ΔS° for the decomposition
Q14: The value of ΔH° for the decomposition
Q15: The value of ΔH° for the oxidation
Q16: For the reaction C(s)+ <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="For