Multiple Choice
The half-reaction occurring at the cathode in the balanced reaction shown below is ________. 3MnO4- (aq) + 24H+ (aq) + 5Fe (s) → 3Mn2+ (aq) + 5Fe3+ (aq) + 12H2O (l)
A) MnO4- (aq) + 8H+ (aq) + 5 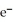 → Mn2+ (aq) + 4H2O (l)
→ Mn2+ (aq) + 4H2O (l)
B) 2MnO4- (aq) + 12H+ (aq) + 6e- → 2Mn2+ (aq) + 3H2O (l)
C) Fe (s) → Fe3+ (aq) + 3e-
D) Fe (s) → Fe2+ (aq) + 2e-
E) Fe2+ (aq) → Fe3+ (aq) + e-
Correct Answer:

Verified
Correct Answer:
Verified
Q53: Which transformation could take place at the
Q54: What is the anode in an alkaline
Q55: What is the oxidation number of sulfur
Q56: Which transformation could take place at the
Q57: In the electrochemical cell using the redox
Q59: In the galvanic cell using the redox
Q60: What is the oxidation number of phosphorous
Q61: The lead-containing reactant(s)consumed during recharging of a
Q62: Which element is oxidized in the following
Q63: Which one of the following reactions is