Multiple Choice
The relationship between the change in Gibbs free energy and the emf of an electrochemical cell is given by ________.
A) ΔG = 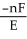
B) ΔG = 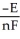
C) ΔG = -nFE
D) ΔG = -nRTF
E) ΔG = 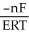
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q81: The _ of the alkaline battery is
Q82: What is the oxidation number of manganese
Q83: _ electrons appear in the following half-reaction
Q84: The standard reduction potential,E°<sub>red</sub>,is proportional to the
Q85: _ is the oxidizing agent in the
Q87: The balanced half-reaction in which dichromate ion
Q88: Which element is oxidized in the reaction
Q89: How many grams of Ca metal are
Q90: The amount of a substance that is
Q91: Consider an electrochemical cell based on the