Multiple Choice
The standard cell potential (E°cell) of the reaction below is -0.55 V.The value of 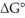 for the reaction is ________ J/mol. I2 (s) + 2Br- (aq) → 2I- (aq) + Br2 (l)
for the reaction is ________ J/mol. I2 (s) + 2Br- (aq) → 2I- (aq) + Br2 (l)
A) 0.54
B) 0.55
C) 5.5 × 10-6
D) 1.1 × 105
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q71: The standard emf for the cell using
Q72: The electrode where reduction occurs is called
Q73: Which element is oxidized in the reaction
Q74: Which element is reduced in the following
Q75: The balanced half-reaction in which ethanol,CH<sub>3</sub>CH<sub>2</sub>OH,is oxidized
Q77: Which element is oxidized in the reaction
Q78: In a half reaction,the amount of a
Q79: Which one of the following is the
Q80: Which substance is the oxidizing agent in
Q81: The _ of the alkaline battery is