Multiple Choice
The standard cell potential (E°cell) of the reaction below is -0.34 V.The value of 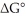 for the reaction is ________ kJ/mol. Cu (s) + 2H+ (aq) → Cu2+ (aq) + H2 (g)
for the reaction is ________ kJ/mol. Cu (s) + 2H+ (aq) → Cu2+ (aq) + H2 (g)
A) -0.34
B) +66
C) -130
D) +130
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: What is the oxidation number of oxygen
Q2: The major product of a _ fuel
Q3: Which substance does not undergo oxidation or
Q4: What is the oxidation number of manganese
Q5: The lithium ion battery has more energy
Q7: Which transformation below is an example of
Q8: The standard cell potential ( <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg"
Q9: Which substance is the oxidizing agent in
Q10: Which substance is the oxidizing agent in
Q11: In a voltaic cell electrons flow from