Multiple Choice
How many seconds are required to produce 5.00 g of aluminum metal from the electrolysis of molten 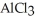 with an electrical current of 15.0 A?
with an electrical current of 15.0 A?
A) 27.0
B) 9.00
C) 1.19E × 103
D) 2.90 × 105
E) 3.57 × 103
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q61: The lead-containing reactant(s)consumed during recharging of a
Q62: Which element is oxidized in the following
Q63: Which one of the following reactions is
Q64: Which element is oxidized in the reaction
Q65: Galvanized iron is iron coated with _.<br>A)magnesium<br>B)zinc<br>C)chromium<br>D)phosphate<br>E)iron
Q67: Which one of the following types of
Q68: The standard reduction potential of X is
Q69: The reduction half reaction occurring in the
Q70: How many minutes will it take to
Q71: The standard emf for the cell using