Multiple Choice
How many seconds are required to produce 1.0 g of silver metal by the electrolysis of a AgN 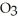 solution using a current of 60 amps?
solution using a current of 60 amps?
A) 5.4 × 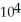
B) 3.2 × 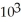
C) 15
D) 3.7 × 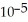
E) 30
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q25: One of the differences between a voltaic
Q26: The standard cell potential (E°<sub>cell</sub>)of the reaction
Q27: What is the oxidation number of oxygen
Q28: Which of the statements about cathodic protection
Q29: What is the coefficient of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg"
Q31: What is the cathode in an alkaline
Q32: What is a galvanized iron nail?
Q33: What is being reduced at the cathode
Q34: Which of the following reactions will occur
Q35: The standard cell potential (E°<sub>cell</sub>)for the voltaic