Multiple Choice
What is the mass defect (in amu) of a 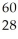 Ni nucleus if the nuclear mass is 59.9308 amu? The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 amu.
Ni nucleus if the nuclear mass is 59.9308 amu? The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 amu.
A) 0.5449
B) 0.5505
C) 1.2374
D) 1.3066
E) 28.7930
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q92: The missing product from this reaction is
Q93: The half-life of <sup>222</sup>Rn is 3.80 days.If
Q94: How much energy (in J)is produced when
Q95: Cesium-131 has a half-life of 9.7 days.What
Q96: The missing product from this reaction is
Q98: The use of radioisotopes in tracing metabolism
Q99: Cobalt-60 is produced by a three reaction
Q100: What percentage of a radioactive sample remains
Q102: What happens to the mass number and
Q136: This reaction is an example of _.