Multiple Choice
Carbon-11 decays by positron emission.The decay occurs with a release of 2.87 × 1011 J per mole of carbon-11.What mass (g) is converted to energy when 5.00 g of 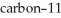 undergoes this radioactive decay?
undergoes this radioactive decay?
A) 1.45 × 10-6
B) 4.35 × 105
C) 6.90 × 102
D) 1.45 × 10-3
E) 1.59 × 10-2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: The three radioactive series that occur in
Q5: What is the missing product from this
Q6: What is the mass number of a
Q7: What is the age in years of
Q8: Carbon-11,fluorine-18,oxygen-15 and nitrogen-13 are all used in
Q10: A _ is a highly reactive substance
Q11: The formation of krypton from rubidium decay
Q12: Of the following processes,which one changes the
Q13: In terms of binding energy per nucleon,what
Q14: What is the rate constant for the