Multiple Choice
Which of the following would produce an acidic solution?
A) Na2O and MgO
B) Na2O, MgO, and CaH2
C) 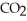 only
only
D) CaH2 only
E) CO, CO2, and CaH2
Correct Answer:

Verified
Correct Answer:
Verified
Q157: What is the function of the carbon
Q158: The interhalogen compound ICl<sub>3</sub> can form but
Q159: The disilicate ion is _.<br>A)Si<sub>2</sub>O<sub>8</sub><sup>8-</sup><br>B)Si<sub>2</sub>O<sub>7</sub><sup>6-</sup><br>C)Si<sub>2</sub>O<sub>8</sub><sup>4-</sup><br>D)Si<sub>2</sub>O<sub>8</sub><sup>6-</sup><br>E)Si<sub>2</sub>O<sub>7</sub><sup>2-</sup>
Q160: The two allotropic forms of phosphorus are
Q161: The instability of xenon fluorides is due
Q163: Which halogen forms an oxyacid with the
Q164: The oxidation number of N in NO<sub>2</sub><sup>-
Q165: The active ingredient in many liquid bleaches
Q166: The correct name for NaBH<sub>4</sub> is _.<br>A)sodium
Q167: Photochemical decomposition of HNO<sub>3</sub> produces small amounts