Multiple Choice
Which of the following equations correctly represents the combustion of hydrazine?
A) N2H4 (l) + O2 (g) → NH3 (g) + HNO2 (g)
B) N2H4 (l) + 2O2 (g) → 2NO2 (g) + 2H2 (g)
C) N2H4 (l) + O2 (g) → 2 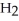 NO (g)
NO (g)
D) N2H4 (l) + O2 (g) → N2 (g) + 2 H2O (g)
E) N2H4 (l) + O2 (g) → N2 (g) + 2H2 (g) + O2 (g)
Correct Answer:

Verified
Correct Answer:
Verified
Q103: The oxidation state of oxygen in O<sub>2</sub>F<sub>2</sub>
Q104: Chlorine can have a positive oxidation state
Q105: What are the five crystalline allotropes of
Q106: What is the F-Xe-F bond angle in
Q107: Hybridization of Xe in XeF<sub>4</sub> is _
Q109: Low levels of arsenic consumption can lead
Q110: Which of the following is the nitride
Q111: Amphoteric oxides are also known as _.<br>A)basic
Q112: H<sub>2</sub> is reacted with _ to produce
Q113: All oxides are ionic compounds.