Multiple Choice
Sodium borohydride,NaB 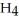 ,is a strong reducing agent because ________.
,is a strong reducing agent because ________.
A) Na+ is easily reduced to Na (s)
B) boron easily changes its oxidation number from +3 to -3
C) boron is readily oxidized from -3 oxidation state to +3
D) hydrogen can be easily oxidized from -1 oxidation state to +1
E) hydrogen is easily reduced from +1 oxidation state to 0
Correct Answer:

Verified
Correct Answer:
Verified
Q67: What noble gas is radioactive?
Q68: In a proton transfer reaction the weaker
Q69: Oxides can react with water to form
Q70: What are the principal components used in
Q71: What is the molecular shape of the
Q73: Group 8A elements are all gases at
Q74: The Ostwald process is by which NH<sub>3</sub>
Q75: The acid and salts of which halogen-oxyanion
Q76: The oxidation state of fluorine in its
Q77: Which halogen can react with fluorine to