Multiple Choice
Which of the following equations correctly represents the reaction of 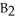
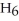 with oxygen?
with oxygen?
A) B2H6 (g) + 3O2 (g) → B2O3 (s) + 3H2O (g)
B) B2H6 (g) + O2 (g) → B2O2 (s) + 3H2 (g)
C) B2H6 (g) + 2O2 (g) → B2H2 (s) + 2H2O2 (aq)
D) B2H6 (g) + 2O2 (g) → B2O2 (s) + 3H2 + O2 (g)
E) B2H6 (g) + O2 (g) → H2B2O2 (s) + 2H2 (g)
Correct Answer:

Verified
Correct Answer:
Verified
Q126: Which of the following compounds would produce
Q127: To produce carbon black,_.<br>A)diamond is exposed to
Q128: _ is formed when wood is heated
Q129: Of the atoms below,_ is the most
Q130: An aqueous solution of HF is considered
Q132: What method is used to produce the
Q133: The electrical conductivity of _ is low
Q134: The danger from mixing ammonia with bleach
Q135: The heavier noble gases are more reactive
Q136: The primary commercial use of elemental nitrogen