Multiple Choice
Which equation correctly represents the reaction between elemental chlorine and sodium iodide?
A) Cl + NaI → I + NaCl
B) 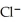 + NaI →
+ NaI → 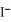 + NaCl
+ NaCl
C) 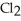 + 2NaI →
+ 2NaI → 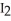 + 2NaCl
+ 2NaCl
D) Cl + NaI → 1/2 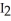 + NaCl
+ NaCl
E) 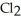 + NaI → Na
+ NaI → Na 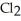 +
+ 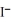
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q167: Photochemical decomposition of HNO<sub>3</sub> produces small amounts
Q168: Hydrogen peroxide is a highly polar and
Q169: What process replenishes O<sub>2</sub>?
Q170: Which compound would produce a basic aqueous
Q171: What anion containing Cl is used as
Q173: The oxidation state of sulfur in the
Q174: Additives can be used in soda-lime glass
Q175: What are the products of the reaction
Q176: The number of electrons in the valence
Q177: What is the oxidation state of oxygen