Multiple Choice
The following figure shows a(an) : 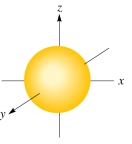
A) d orbital
B) p orbital
C) s orbital
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q31: All of the following are used to
Q32: Which element has a ground state electron
Q33: How many sublevels are occupied in an
Q34: How many orbitals are contained in the
Q35: The second principal energy level contains four
Q37: On the periodic table,elements that behave in
Q38: List the following orbitals in order of
Q39: How many valence electrons are present in
Q40: The number of waves that pass a
Q41: All noble gases have eight electrons in