Multiple Choice
The following figure represents one mole of an ideal gas in a container fit with a movable piston. 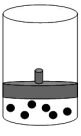 Which figure shows the change,if any,that would take place if the Kelvin temperature is doubled under constant pressure?
Which figure shows the change,if any,that would take place if the Kelvin temperature is doubled under constant pressure?
A) 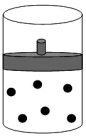
B) 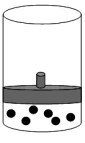
C) 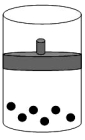
D) 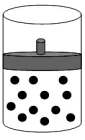
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q53: What mass of water is produced
Q54: The density of an unknown gas at
Q55: A pressure of 1030 torr is equal
Q56: Which gas will diffuse most rapidly?<br>A)CO<sub>2</sub><br>B)N<sub>2</sub><br>C)He<br>D)Cl<sub>2</sub>
Q57: What is the molar mass of a
Q59: A 250.mL sample of methane gas is
Q60: The following figure shows 1 mol of
Q61: Under which set of conditions will a
Q62: At which pressure would a 2.60 mole
Q63: A 400.mL sample of hydrogen is collected