Multiple Choice
What is the pH of a solution whose H3O +1 concentration is 1 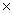 10 -9?
10 -9?
A) 1
B) 9
C) -9
D) -1
Correct Answer:

Verified
Correct Answer:
Verified
Q47: Which are the two Bronsted-Lowry acids in
Q48: What is the freezing point of a
Q49: Calculate the pH of the following solutions:<br>A.0.034
Q50: Which pH is neutral?<br>A)2<br>B)4<br>C)5<br>D)7
Q51: In an acidic solution the<br>A)concentration of hydronium
Q53: Strong acids are strong electrolytes.
Q54: What is the concentration of calcium ion
Q55: Which is a strong base?<br>A)NH<sub>4</sub>OH<br>B)Ca(OH)<sub>2</sub><br>C)HNO<sub>2</sub><br>D)HNO<sub>3</sub>
Q56: Which is a strong acid?<br>A)HClO<sub>3</sub><br>B)HClO<sub>2</sub><br>C)H<sub>2</sub>SO<sub>3</sub><br>D)H<sub>2</sub>SO<sub>4</sub>
Q57: For the following neutralization reaction:<br>hydrochloric acid plus