Multiple Choice
What is the pH of a solution whose H +1 concentration is 4.0 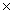 10 -9?
10 -9?
A) 4.0
B) 8.4
C) 3.6
D) 9.0
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q69: What is the boiling point of a
Q70: Which type of mixture displays Brownian motion
Q71: Which type of compound is not an
Q72: What is the conjugate acid of NH<sub>3</sub>?<sup>
Q73: What is the pH of a 1.0
Q75: Which acid and base will combine to
Q76: A hydrogen ion consists of a(n)<br>A)electron.<br>B)proton.<br>C)neutron.<br>D)proton and
Q77: Which is a conjugate acid base pair
Q78: Acids react with active metals to produce
Q79: The following substances are Lewis bases: ammonia,water,and