True/False
At 25 °C the concentration of H +1 in pure water is 1 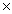 10 -7 M.
10 -7 M.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q26: Which is a conjugate acid base pair
Q27: What mass of sodium chloride should be
Q28: Which is a strong electrolyte?<br>A)water<br>B)acetic acid<br>C)ammonia<br>D)sodium chloride
Q29: What is the conjugate base of NH<sub>3</sub>?<br>A)NH<sub>2</sub><sup>
Q30: Which pH is most alkaline?<br>A)1<br>B)5<br>C)7<br>D)12
Q32: Which are the two Bronsted-Lowry bases in
Q33: Combining potassium hydroxide and sulfuric acid will
Q34: Which pH is most acidic?<br>A)3<br>B)7<br>C)9<br>D)14
Q35: What is the conjugate acid of OH<sup>
Q36: What is the concentration of chloride ion