True/False
The net ionic equation for the neutralization of nitric acid by potassium hydroxide is 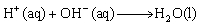 .
.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q34: Which pH is most acidic?<br>A)3<br>B)7<br>C)9<br>D)14
Q35: What is the conjugate acid of OH<sup>
Q36: What is the concentration of chloride ion
Q37: Which are the two Bronsted-Lowry bases in
Q38: What is the concentration of a HNO<sub>3</sub>
Q40: In the following reaction, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4036/.jpg" alt="In
Q41: A hydronium ion is a hydrated proton.
Q42: What is the pH of a 0.020
Q43: What is the pH of a 0.01
Q44: A base solution contains 0.400 moles of