True/False
An isotope of gallium consisting of 31 protons and 37 neutrons can be represented using the symbol shown below. 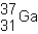
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: What is the molar mass of Ba<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub>?<br>A)232.30
Q4: H<sub>2</sub>O<sub>2</sub> contains equal parts by weight of
Q8: The weight % of S in K<sub>2</sub>SO<sub>4</sub>
Q18: An MRI instrument cause the hydrogens in
Q20: One mole of H<sub>2</sub>O contains 2.0 grams
Q32: The mass of mercury (Hg), a liquid
Q48: If you have 3.011 *10<sup>23</sup> atoms of
Q64: Isotopes of the same element always have
Q75: Calculate the weight percentage of hydrogen in
Q87: Electrons do not make an important contribution