Multiple Choice
A solution is prepared at 75 °C by dissolving 50.0 g of A in 100 g of water.When this solution is cooled to 15 °C what happens based on the following solubility plot for A. 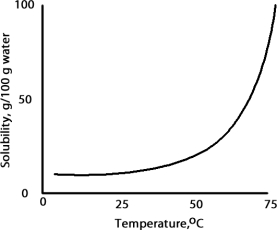
A) Solid A crystallizes and the solution is unsaturated.
B) Solid A dissolves and the solution is supersaturated.
C) Solid A crystallizes and the solution is saturated.
D) Solid A dissolves and the solution is unsaturated.
Correct Answer:

Verified
Correct Answer:
Verified
Q22: The primary intermolecular attractions between CH<sub>3</sub>-OH and
Q30: The freezing point of a 0.500 M
Q39: How many grams of solid KCl are
Q45: Putting a celery stick in distilled water
Q49: When an ionic substance dissolves, the solvated
Q67: Attractive forces between solute and solvent molecules
Q70: What is the molarity of a solution
Q71: The solubility of a substance can be
Q71: The %(w/v)of a solution is defined as:
Q95: The rate of osmosis<br>A)can be increased by