Multiple Choice
The theorem of equipartition of energy states that the energy each degree of freedom contributes to each molecule in the system (an ideal gas) is
A) 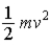 .
.
B) 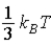 .
.
C) 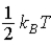 .
.
D) 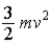 .
.
E) 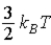 .
.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q5: The molar specific heat at constant volume
Q6: Nitrogen gas is heated by a pulsed
Q8: If the rms speed of helium atoms
Q11: The average molecular translational kinetic energy of
Q12: The relation PV = nRT holds for
Q14: The molar specific heat at constant volume
Q15: The molar specific heat at constant pressure
Q32: One mole of helium gas expands adiabatically
Q35: The average kinetic energy of a nitrogen
Q36: Assume molecules have an average diameter of