Multiple Choice
The K,L,M symbols represent values of the quantum number
A) n
B) 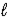
C) 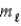
D) ms
E) mj
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: All quantum states forming a sub-shell have
Q9: Zeke says that the magnitude of the
Q12: An electron is moving at a speed
Q26: The energy difference between the upper and
Q30: Light is emitted by hydrogen atoms in
Q35: An electron in a hydrogen atom makes
Q37: How fast is the electron moving in
Q39: Suppose a beam of electrons is incident
Q43: Suppose Bohr had chosen the potential energy
Q44: The ground state configuration of chlorine (Z