Multiple Choice
In the Bohr model of the hydrogen atom,the total energy of the electron-proton system is
A) 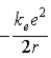 .
.
B) 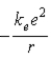 .
.
C) 0.
D) 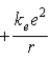 .
.
E) 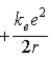 .
.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: In 1921, Stern and Gerlach performed an
Q10: What wavelength (in μm) is associated with
Q16: What is the difference in frequency for
Q28: The magnitude of the spin angular momentum
Q31: The radial portion of the de Broglie
Q32: In an allowed electron transition in a
Q34: The probability density for the 1s state
Q37: Which of the following,in which n and
Q41: An electron in a hydrogen atom makes
Q55: What is the difference in wavelength for