Multiple Choice
The number of electrons in the n = 4, 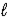
= 2 subshell in strontium (Z = 38) is ____ the number of electrons in the n =4, 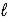
=2 subshell in barium (Z = 56) .
A) 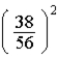 times
times
B) 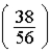 times
times
C) equal to
D) 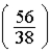 times
times
E) 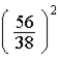 times
times
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: For the following allowed transitions, which photon
Q15: The Pauli Exclusion Principle states<br>A) no two
Q16: What is the difference in frequency for
Q21: In a shell of the hydrogen atom
Q28: The magnitude of the spin angular momentum
Q31: The radial portion of the de Broglie
Q38: Characteristic x-rays can be produced by bombarding
Q41: An electron in a hydrogen atom makes
Q45: An energy of 13.6 eV is needed
Q51: The energy needed to change a He<sup>+</sup>