Multiple Choice
All quantum states forming a shell have the same
A) principal quantum number n.
B) orbital quantum number 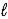 .
.
C) orbital magnetic quantum number 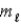 .
.
D) n, 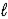 and
and 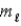
E) n and 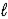 only.
only.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: When electrons fill a subshell in which
Q8: Of the following states, 5s, 3p, 4f,
Q12: In the subshell of the Li<sup>2+</sup> ion
Q14: The s,p,d,f,symbols represent values of the quantum
Q17: The allowed values of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2295/.jpg" alt="The
Q20: Adam and Eve are contemplating the beauty
Q21: In a shell of the hydrogen atom
Q35: An electron in a hydrogen atom makes
Q36: In a completely filled atomic shell,<br>A) the
Q40: A hydrogen atom is in its first