Multiple Choice
The diagram below shows the distance between the nuclei,pA and pB,and the electrons,e1 and e2,in a hydrogen molecule.We would expect the electrostatic potential energy of this molecule to be 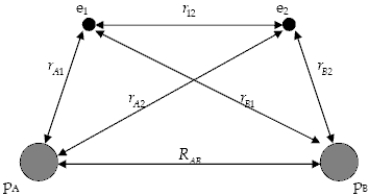
A) 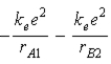 .
.
B) 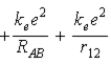 .
.
C) 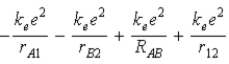 .
.
D) 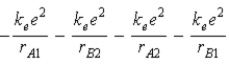 .
.
E) 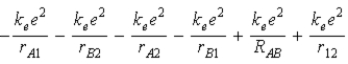 .
.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: When a molecule jumps from a rotational
Q4: What is the energy of the first
Q5: An experiment determines that there are 49
Q8: The rotation spectrum of the HCl molecule
Q9: To find the number of electrons per
Q11: When a molecule jumps from a rotational
Q27: An LED emits light of wavelength 600
Q38: Solid argon has a density of 1650
Q39: The Fermi temperature of copper is 80
Q44: Which of the following refer to the