Multiple Choice
What value of Z (atomic number) and A (mass number) result in the following alpha decay? 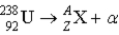
A) Z = 92;A = 238
B) Z = 91;A = 238
C) Z = 90;A = 234
D) Z = 93;A = 238
E) Z = 88;A = 236
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: The ratio of the radius of a
Q7: The isotope, tritium, has a half-life of
Q12: Naturally radioactive nuclei can decay spontaneously by
Q15: Two nuclei which share the same mass
Q19: A glass container holds equal numbers of
Q20: Heavy nuclei are unstable because<br>A) each nucleon
Q22: What is the disintegration energy (in MeV)associated
Q38: It is often possible to use atomic
Q68: Find the binding energy (in MeV) of
Q75: In beta decays<br>A) a proton changes to