Multiple Choice
What value of Z (atomic number) and A (mass number) result in the following β-decay? 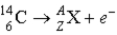
A) Z = 5;A = 14
B) Z = 4;A = 10
C) Z = 6;A = 14
D) Z = 7;A = 14
E) Z = 7;A = 13
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q6: Find the ratio of the binding energy
Q7: What value of Z (atomic number)and A
Q7: The isotope, tritium, has a half-life of
Q11: A pure sample of <sup>226</sup>Ra contains 2.0
Q11: What value of Z (atomic number)and A
Q62: For large mass number nuclei which are
Q69: Because we know that the half-lives of
Q71: Homer says that we can safely use
Q75: In beta decays<br>A) a proton changes to
Q88: Two nuclei may have equal Z, but