Multiple Choice
Which of the following molecules is most likely to show a dipole-dipole interaction?
A) CH3OH
B) CH3SH
C) CH4
D) H-C 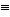 C-H
C-H
E) A and B
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q20: Ammonia, NH<sub>3</sub>, is more polar than is
Q20: How many nonbonding pairs of electrons are
Q24: Which of the following molecules should have
Q26: Magnesium ions carry a 2+ charge, and
Q62: Which of the following statements best describes
Q77: Is an ionic compound an example of
Q87: What is the difference between a dipole-dipole
Q92: Atoms of metallic elements can form ionic
Q94: Which of the following bonds would be
Q97: Which should be larger,the potassium atom,K,or the