Multiple Choice
Consider the boiling points of the following compounds and their solubilities in room-temperature water. Why does the solubilities in water go down as the boiling points of these alcohols go up. 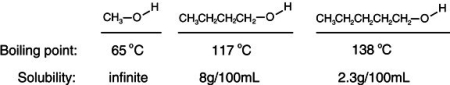
A) Larger molecules are less attracted to one another by induced dipole-induced dipole as well as by dipole-dipole and dipole-induced dipole attractions.
B) As the boiling increases, it is more difficult to keep the alcohol from evaporating out of solution.
C) As the boiling point increases, the size of the alcohol molecules decreases.
D) Larger molecules are more attracted to one another by induced dipole-induced dipole as well as by dipole-dipole and dipole-induced dipole attractions.
Correct Answer:

Verified
Correct Answer:
Verified
Q11: Which would you expect to have a
Q12: Which of the following is the correct
Q16: Which are closer together: the two nuclei
Q63: How many oxide ions (O<sup>-2</sup>)are needed to
Q77: Is an ionic compound an example of
Q92: Atoms of metallic elements can form ionic
Q131: Which of the following molecules has the
Q135: An atom loses an electron to another
Q141: Which of the following molecules contains a
Q149: Metals are useful for the structural support