Multiple Choice
Identify the acid or base behavior of each substance in these reactions: HSO4- + H2O ⇌ OH- + H2S 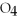
A) HS O4- acts as an acid, H2O acts as a base, OH- acts as an acid, H2S O4 acts as a base.
B) HS O4- acts as an base, H2O acts as a acid, OH- acts as an acid, H2S O4 acts as a base.
C) HS O4- acts as an acid, H2O acts as a base, OH- acts as an base, H2S O4 acts as a acid.
D) HS O4- acts as an base, H2O acts as a acid, OH- acts as an base, H2S O4 acts as a acid.
Correct Answer:

Verified
Correct Answer:
Verified
Q31: Why are combustion reactions generally exothermic?<br>A)Hydrogen easily
Q37: In a battery,the following oxidation-reduction reactions are
Q53: What is a reduction?<br>A)the gain of electrons<br>B)the
Q55: What would the concentration of H<sub>3</sub>O<sup>+</sup> be
Q84: Your car lights were left on while
Q101: A major source of chlorine gas, Cl<sub>2</sub>,
Q104: What would be the concentration of hydronium
Q133: Which of the following describes an electrode?<br>A)Any
Q169: In a battery,the following two oxidation-reduction reactions
Q174: Why might an area with a large