Multiple Choice
For the n = 5 shell,what are the lowest values possible for 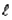
And 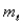
Respectively?
A) -5,-5
B) −4,-4
C) 0,-4
D) 0,0
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q42: The ionization energy of the hydrogen atom
Q43: If the radius of the electron orbit
Q44: The quantum mechanical model of the hydrogen
Q45: In contrast to Thomson's model of the
Q46: The ionization energy of the hydrogen atom
Q48: In a plot of probability of finding
Q49: In the hydrogen atom the potential energy
Q50: The ionization energy for the hydrogen atom
Q51: In the n = 4 shell,how many
Q52: The stimulated emission of photons from the