Multiple Choice
For which of the following transitions in a hydrogen atom free of external fields does the electron energy increase?
A) The electron moves from n = 2 to n = 1.
B) The electron moves from n = 3 to n = 5.
C) Within the n = 4,l = 2 sublevel,the electron moves from  = -1 to
= -1 to  = +1.
= +1.
D) Within the n = 3 level,the electron moves from 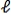 = 2 to
= 2 to 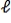 = -2.
= -2.
Correct Answer:

Verified
Correct Answer:
Verified
Q19: The four visible colors emitted by hydrogen
Q20: When a wire carries high current causing
Q21: What is the energy needed to change
Q22: Of the various wavelengths emitted from a
Q23: An energy of 13.6 eV is needed
Q25: Characteristic K x-rays are the result of:<br>A)inner
Q26: The quantum mechanical model of the hydrogen
Q27: The quantum mechanical model of the hydrogen
Q28: In an x-ray machine,electrons are accelerated and
Q29: The Paschen series of hydrogen corresponds to