Multiple Choice
Sample #1 is made from an isotope with decay constant 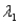
And sample #2 is made from an isotope with decay constant 
,where 
) Which of the following statements must be true?
A) The activity of sample #2 is greater than that of sample #1.
B) The activity of sample #1 is greater than that of sample #2.
C) The half-life exhibited for sample #1 is greater than that of sample #2.
D) The half-life exhibited for sample #2 is greater than that for sample #1
Correct Answer:

Verified
Correct Answer:
Verified
Q16: The isotope <sup>14</sup>C cannot be used in
Q17: The fact that the binding energy per
Q18: In the four radioactive series,the nuclei decay
Q19: An alpha particle (mass = 6.68 ×
Q20: When radium-224 emits an alpha particle,the remaining
Q22: A deuteron is captured by an oxygen-16
Q23: If there are 146 neutrons in <sup>238</sup>U,how
Q24: Approximately how many radioactive atoms are present
Q25: Rutherford's experiments involving the use of alpha
Q26: What is the energy released for the