Multiple Choice
You take 50 mL of small BB's and combine them with 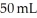 of large BB's and you get a total of 90 mL of BB's of mixed size. Which of the following statements best explains this?
of large BB's and you get a total of 90 mL of BB's of mixed size. Which of the following statements best explains this?
A) Since the density of the small BB's is less than that of the large BB's their volumes do not add directly to one another.
B) This is not possible since the Law of Conservation of Volume would be violated.
C) The total volume actually gets larger since mixing the BB's would leave additional air space because of the difference in size of the two BB sets.
D) Many of the smaller BB's are able to fit within the pockets of space that were empty within the 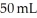 of large BB's.
of large BB's.
Correct Answer:

Verified
Correct Answer:
Verified
Q3: Imagine that you can see individual molecules.
Q24: With increasing temperature the density of air
Q43: The value 300 joules could be a
Q51: When you suck through a soda straw
Q51: How many milliliters of air are there
Q53: At a depth of about 10 meters
Q65: If the density of a block of
Q78: Which of the following is an example
Q84: Alchemist of the Middle Ages believed that
Q99: Gas particles travel at speeds of up