Multiple Choice
Which has stronger attractions among its submicroscopic particles: a solid at 25°C or a gas at 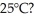
A) The attractions among the submicroscopic particles of the solid must be stronger than the attractions among the particles of the gas.
B) The submicroscopic particles of the gas are moving faster. This means that they have more energy, which means that the attractions among them must be stronger.
C) The attractions among the submicroscopic particles of the solid are much stronger, so much stronger that they hold the particles of the solid absolutely still.
D) The temperatures of these two materials are the same, which means that the attractions among their submicroscopic particles are also of the same strength.
Correct Answer:

Verified
Correct Answer:
Verified
Q5: Dalton's atomic model gained credibility because _.<br>A)atoms
Q6: Which of the following best describes a
Q19: Why is it not possible for the
Q35: Which temperature is the hottest?<br>A)100°C<br>B)100 K<br>C)100°F<br>D)They are
Q35: The following three boxes represent the number
Q36: The diagram on the far left shows
Q46: If the density of mercury is 13.6
Q48: What volume of water would a 52.3-gram
Q53: Which of the following is the largest
Q97: Which of the following does not describe