Multiple Choice
What is the hydroxide ion concentration in an aqueous solution when the hydronium ion concentration equals 1 × 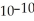 moles per liter?
moles per liter?
A) The concentration of hydroxide ions is 1 × 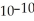 moles per liter.
moles per liter.
B) The concentration of hydroxide ions is 1 × 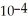 moles per liter.
moles per liter.
C) The concentration of hydroxide ions is 1 × 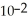 moles per liter.
moles per liter.
D) The concentration of hydroxide ions is 1 × 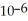 moles per liter.
moles per liter.
Correct Answer:

Verified
Correct Answer:
Verified
Q5: What would the concentration of OH<sup>-</sup> be
Q28: If the pH of a solution was
Q46: Why is the H-F bond so much
Q50: Does a water molecule become more or
Q52: Which of the following items would you
Q52: How might you tell whether or not
Q87: In the reaction below,what does the symbol
Q90: What is an acid?<br>A)anything that donates hydrogen
Q105: According to the following reaction,which molecule is
Q121: In the following buffer system, what happens