Multiple Choice
When a hydronium ion concentration equals 1 × 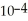 moles per liter, what is the pH of the solution? Is the solution acidic or basic?
moles per liter, what is the pH of the solution? Is the solution acidic or basic?
A) The pH is of this solution is 4 and it is acidic.
B) The pH is of this solution is 8 and it is basic.
C) The pH is of this solution is 6 and it is acidic.
D) The pH is of this solution is 10 and it is basic.
Correct Answer:

Verified
Correct Answer:
Verified
Q5: What would the concentration of OH<sup>-</sup> be
Q46: Why is the H-F bond so much
Q50: Does a water molecule become more or
Q52: How might you tell whether or not
Q55: What would the concentration of H<sub>3</sub>O<sup>+</sup> be
Q57: What atom in the ammonium ion, <img
Q74: What best describes what happens when an
Q77: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6597/.jpg" alt=" -Which of the
Q90: What is an acid?<br>A)anything that donates hydrogen
Q117: A weak acid is added to a